32+ first order reaction calculator
Web First order Differential Equations Calculator. Web Calculate chemical reactions step-by-step.
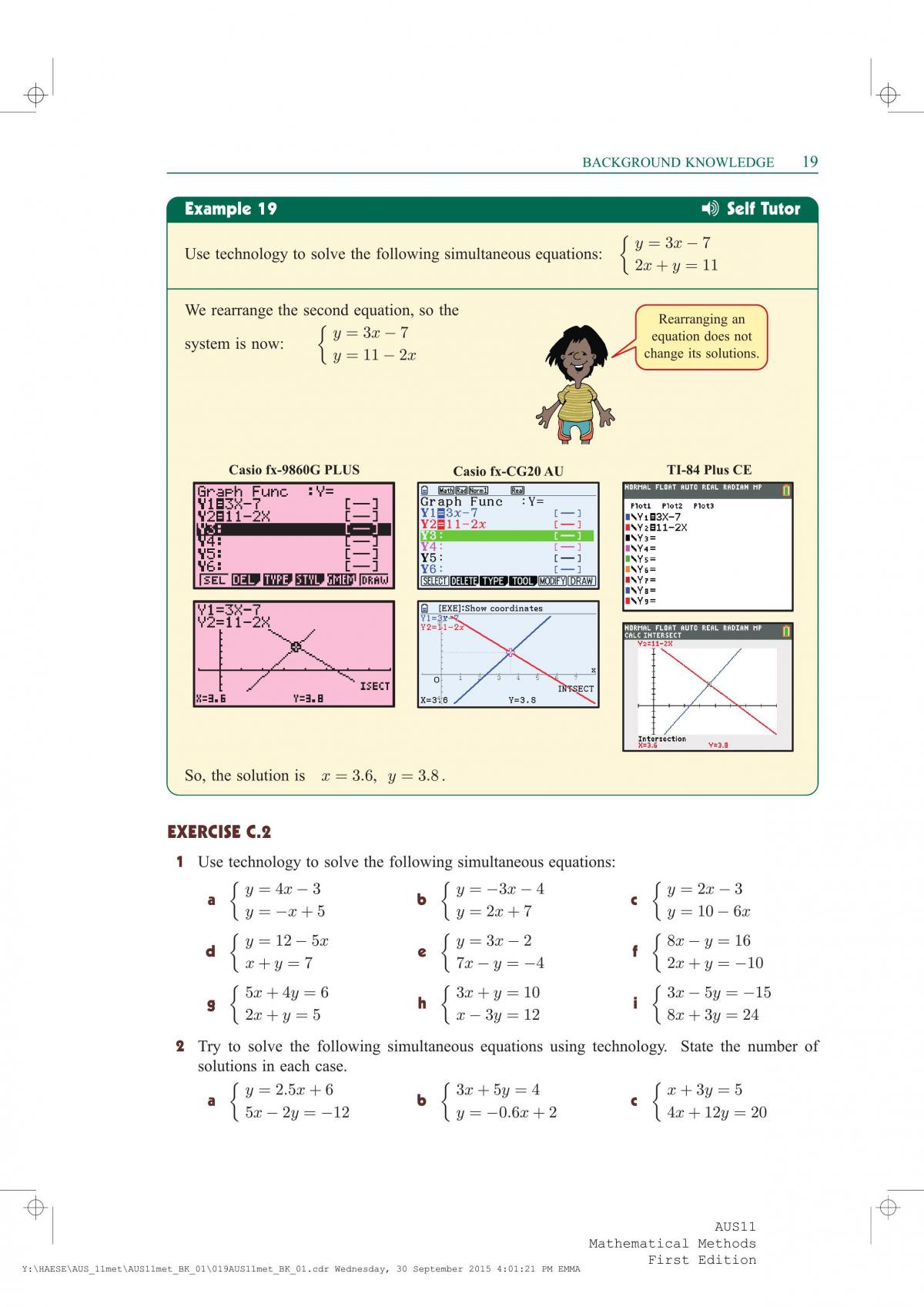
Background Knowledge Mathematical Methods Year 11 Sace Thinkswap
Web Half Life Calculator first order reaction input the equations calculated rate constant.
. Web Given a reaction C2H5Br OH- --- C2H5OH Br- has rate law has rate k C2H5Br OH. If you triple the concentration you triple. Web First analyze your query and determine the number of molecules of reactants reacting in the elementary step.
Web Free linear first order differential equations calculator - solve ordinary linear first order differential equations step-by-step Upgrade to Pro Continue to site Solutions. Please use the mathematical. This widget calculates the half life of.
Zero Order Rate Law Integral form Zero Order. Web First Order rate kA1 kA The rate is directly proportional to the concentration. Web First-order Reaction Definition.
Web For a first order reaction. Web Instructions to use calculator. Enter the scientific value in exponent format for example if you have value as 00000012 you can enter this as 12e-6.
Web The overall order of the reaction is found by adding up the individual orders. Added Dec 9 2011 by ebola3 in Chemistry. The relationship can be linear.
Web In reactions involving many compounds equations can be balanced using an algebraic method based on solving a set of linear equations. When C2H5Br 00477 and OH-0100 M the rate of disappearance of ethyl. Web The number 230 is used to convert the natural logarithm in decimal logarithm.
Also make sure that the chemical equation is balanced otherwise. Get detailed solutions to your math problems with our First order Differential Equations step-by-step calculator. Also substitute A 1 2 A 0 2 from the definition.
The order of a reaction is the relationship between the concentration of a reactant or reactants and the rate. Web The reaction orders in a rate law describe the mathematical dependence of the rate on reactant concentrations. Assign variables to each unknown.
Ln A -kt ln A0 the y variable is now ln A and the x variable is still time. If we tried plotting ln A versus time and get a straight line now. For example if the reaction is first order with respect to both A and B a 1 and b 1 the overall.
Ln 10 2303. If you double the concentration you double the rate. Web The Rate Law calculator has rate of reaction functions for Zero Order First Order and Second Order reactions as follows.
Referring to the generic rate law above the reaction is m order.
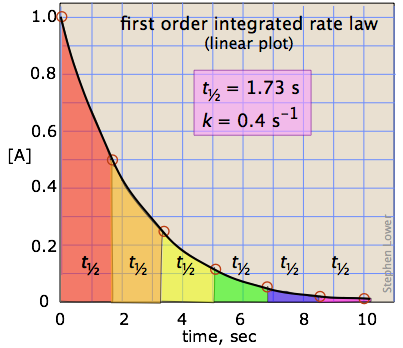
2 3 First Order Reactions Chemistry Libretexts

Calculating The Rate Constant Of A First Order Reaction Youtube
What Is The Cubic Equation Whose Roots Are Alpha Beta And Gamma Quora

Rate Constant Calculator Online Solver With Free Steps
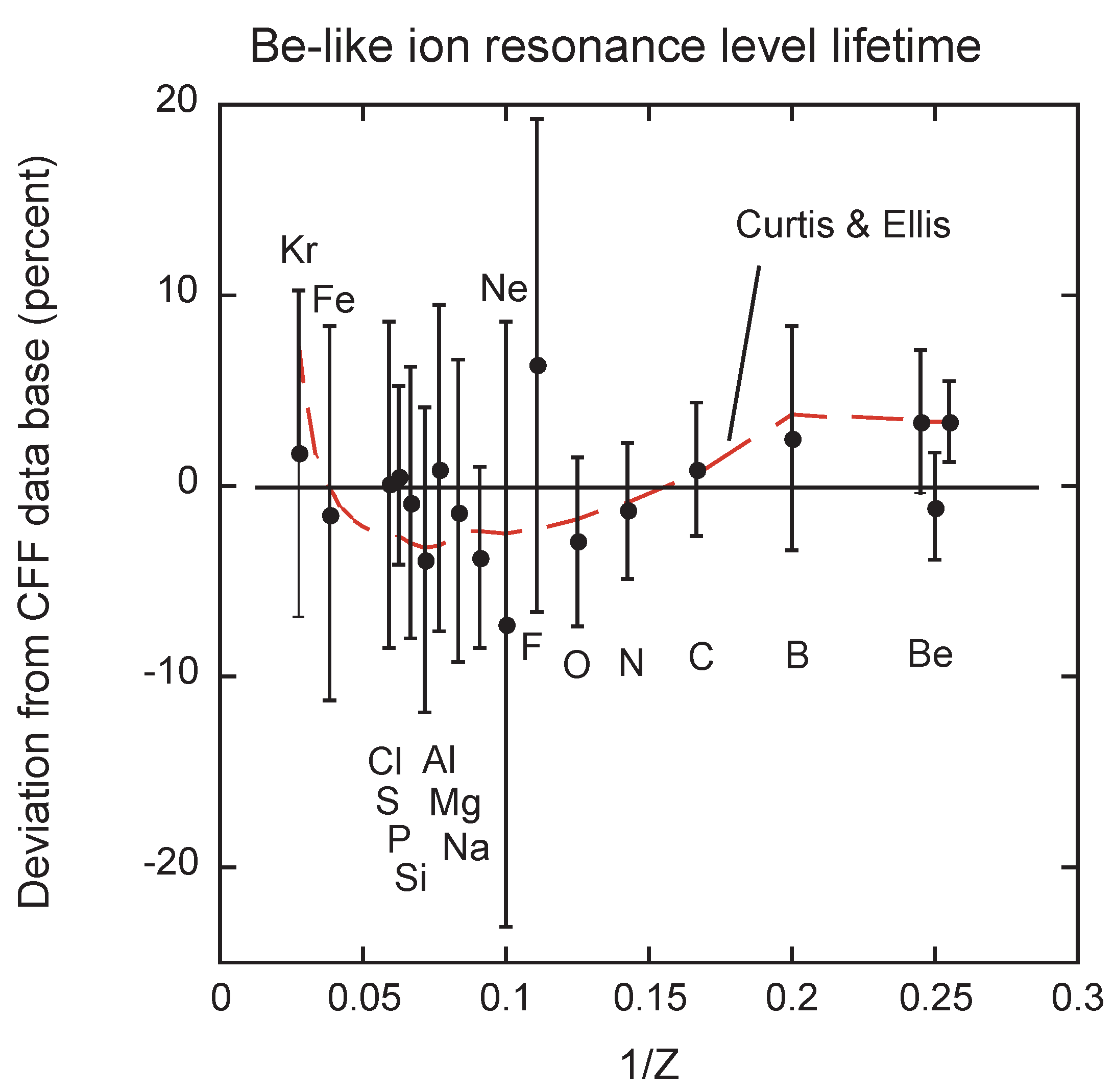
Atoms Free Full Text Critical Assessment Of Theoretical Calculations Of Atomic Structure And Transition Probabilities An Experimenter S View

Medicina Free Full Text A Lower Level Of Post Vaccinal Antibody Titer Against Influenza Virus A H1n1 May Protect Patients With Autoimmune Rheumatic Diseases From Respiratory Viral Infections
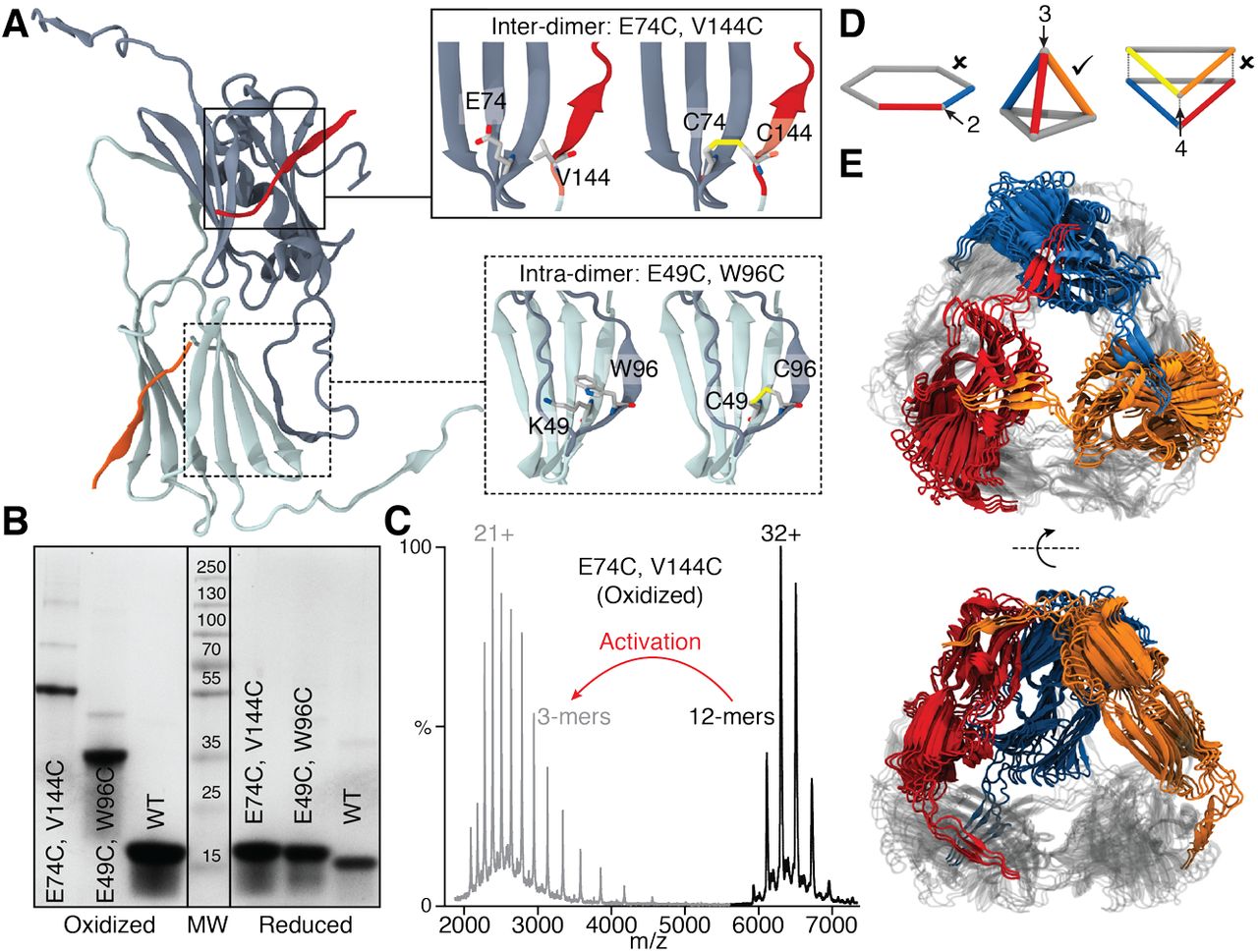
It Takes A Dimer To Tango Oligomeric Small Heat Shock Proteins Dissociate To Capture Substrate Biorxiv
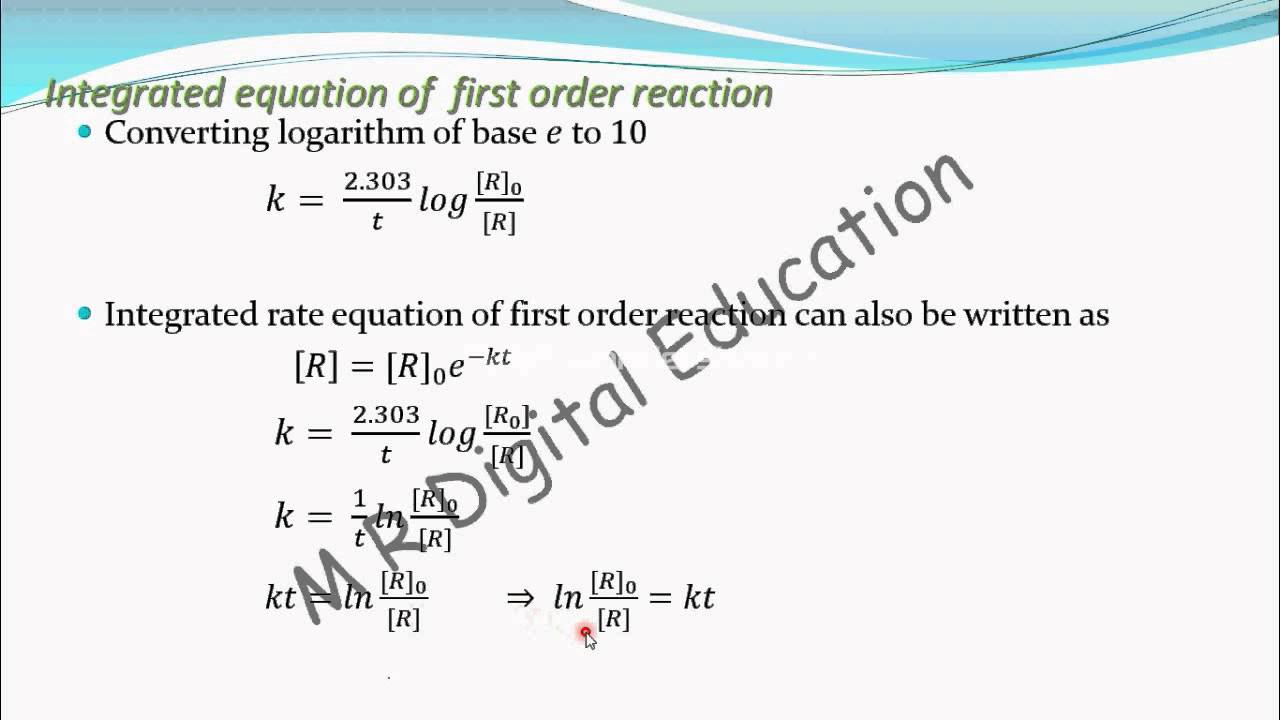
Exponational Form Of Integrated Rate Equation Of First Order Reaction Chemical Kinetics Part 41 Youtube

How To Find The Order Of A Reaction When Rate Constant Is Not Given Chemistry Chemical Kinetics 7131759 Meritnation Com

Rate Of Reaction Calculator Calculator Academy
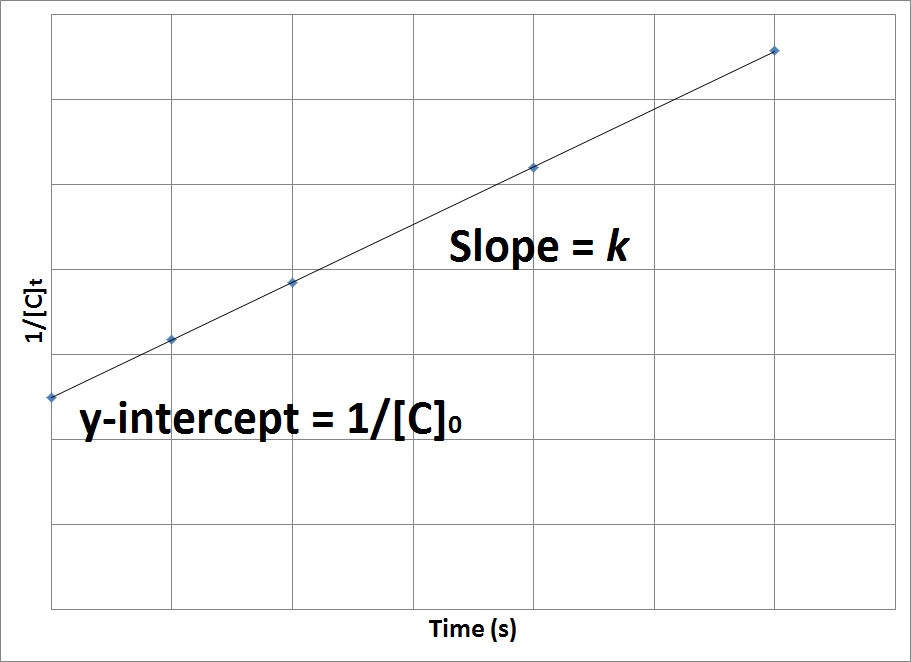
Concentration Time Relationships Integrated Rate Laws Introductory Chemistry 1st Canadian Edition

Key Strategy For The Rational Incorporation Of Long Lived Nir Emissive Cr Iii Chromophores Into Polymetallic Architectures Inorganic Chemistry
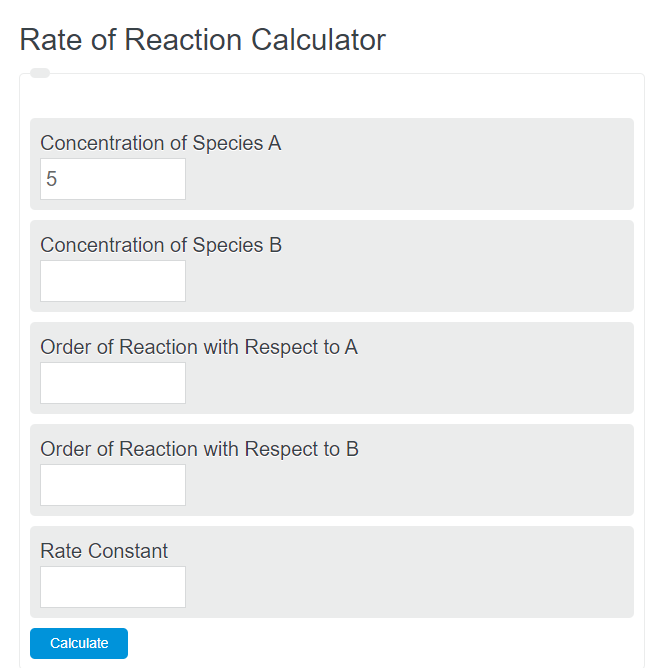
Rate Of Reaction Calculator Calculator Academy

Calculus
Life Is Short And Rom Is Full Issue 110 Numworks Epsilon Github

Fragment Formula Calculator Ffc Determination Of Chemical Formulas For Fragment Ions In Mass Spectrometric Data Analytical Chemistry
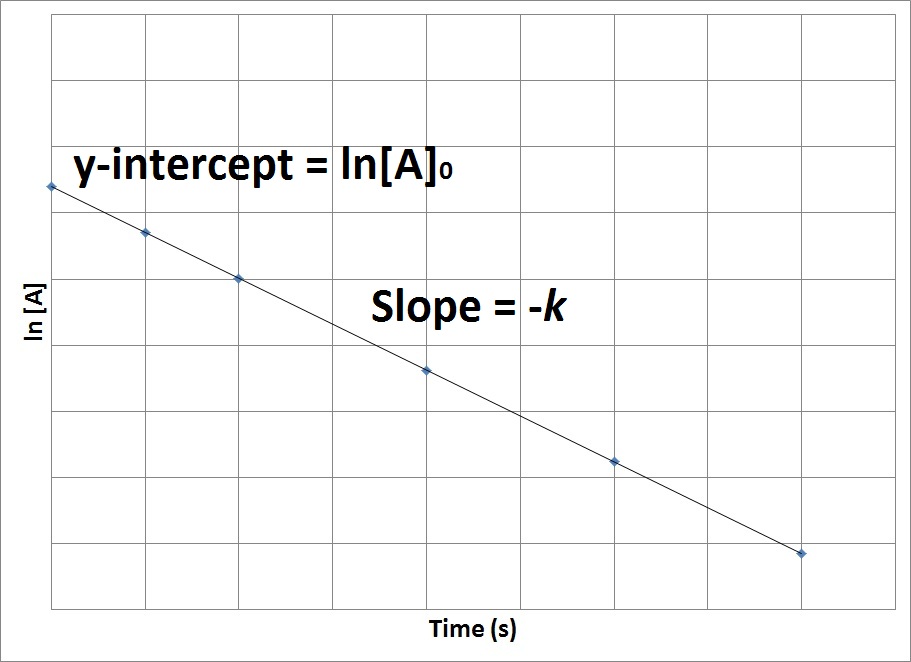
Concentration Time Relationships Integrated Rate Laws Introductory Chemistry 1st Canadian Edition